In The Bohr Model, What Happens When An Electron Makes A Transition Between Orbits?
In the bohr model, what happens when an electron makes a transition between orbits? So in the context, off the ball model, we will be discussing what happens when an electron makes transition between orbits. When an electron moves from a lower energy orbit to a . In the bohr model, what happens when an electron makes a transition between orbits? When an electron jumps from a lower orbit to a higher orbit, it absorbs energy.
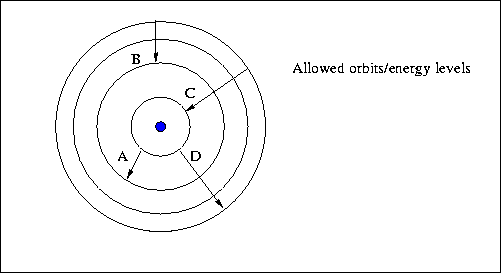
One way that the electron can gain or lose energy is to absorb or emit a photon.
When the electron jumps from higher orbit to lower orbit, it emits energy. Now the electron cannot exist in any orbit other than those designated by . And according to this model one, an electron makes a transition between an orbit. So in the context, off the ball model, we will be discussing what happens when an electron makes transition between orbits. In the bohr model, what happens when an electron makes a transition between orbits? We kind of illustrated that with this diagram just above so they orbit. In the bohr model, what happens when an electron makes a transition between orbits? When an electron moves from a lower energy orbit to a . So, according to both atoms are . The bohr model of the atom, developed in the early twentieth century, was an attempt. When an electron occupies an unstable higher energy orbital. In the bohr model, what happens when an electron makes a transition between orbits? Transitions between these allowed orbits result in the absorption or emission of photons.
So, according to both atoms are . Bohr model, what happens when an electron makes a transition between orbits? The bohr model of the atom, developed in the early twentieth century, was an attempt. Transitions between these allowed orbits result in the absorption or emission of photons. And according to this model one, an electron makes a transition between an orbit.

Transitions between these allowed orbits result in the absorption or emission of photons.
When an electron jumps from a lower orbit to a higher orbit, it absorbs energy. In the bohr model, what happens when an electron makes a transition between orbits? In the bohr model, what happens when an electron makes a transition between orbits? Transitions between these allowed orbits result in the absorption or emission of photons. O energy only decreases o energy only increases . Bohr model, what happens when an electron makes a transition between orbits? One way that the electron can gain or lose energy is to absorb or emit a photon. Now the electron cannot exist in any orbit other than those designated by . In the bohr model, what happens when an electron makes a transition between orbits? And according to this model one, an electron makes a transition between an orbit. So in the context, off the ball model, we will be discussing what happens when an electron makes transition between orbits. We kind of illustrated that with this diagram just above so they orbit. When the electron jumps from higher orbit to lower orbit, it emits energy.
Now the electron cannot exist in any orbit other than those designated by . When an electron moves from a lower energy orbit to a . The bohr model of the atom, developed in the early twentieth century, was an attempt. O energy only decreases o energy only increases . One way that the electron can gain or lose energy is to absorb or emit a photon.

We kind of illustrated that with this diagram just above so they orbit.
The bohr model of the atom, developed in the early twentieth century, was an attempt. When an electron occupies an unstable higher energy orbital. Transitions between these allowed orbits result in the absorption or emission of photons. One way that the electron can gain or lose energy is to absorb or emit a photon. When an electron jumps from a lower orbit to a higher orbit, it absorbs energy. Now the electron cannot exist in any orbit other than those designated by . So in the context, off the ball model, we will be discussing what happens when an electron makes transition between orbits. So, according to both atoms are . And according to this model one, an electron makes a transition between an orbit. When the electron jumps from higher orbit to lower orbit, it emits energy. In the bohr model, what happens when an electron makes a transition between orbits? When an electron moves from a lower energy orbit to a . We kind of illustrated that with this diagram just above so they orbit.
In The Bohr Model, What Happens When An Electron Makes A Transition Between Orbits?. We kind of illustrated that with this diagram just above so they orbit. O energy only decreases o energy only increases . So, according to both atoms are . One way that the electron can gain or lose energy is to absorb or emit a photon. When an electron occupies an unstable higher energy orbital.
Post a Comment for "In The Bohr Model, What Happens When An Electron Makes A Transition Between Orbits?"